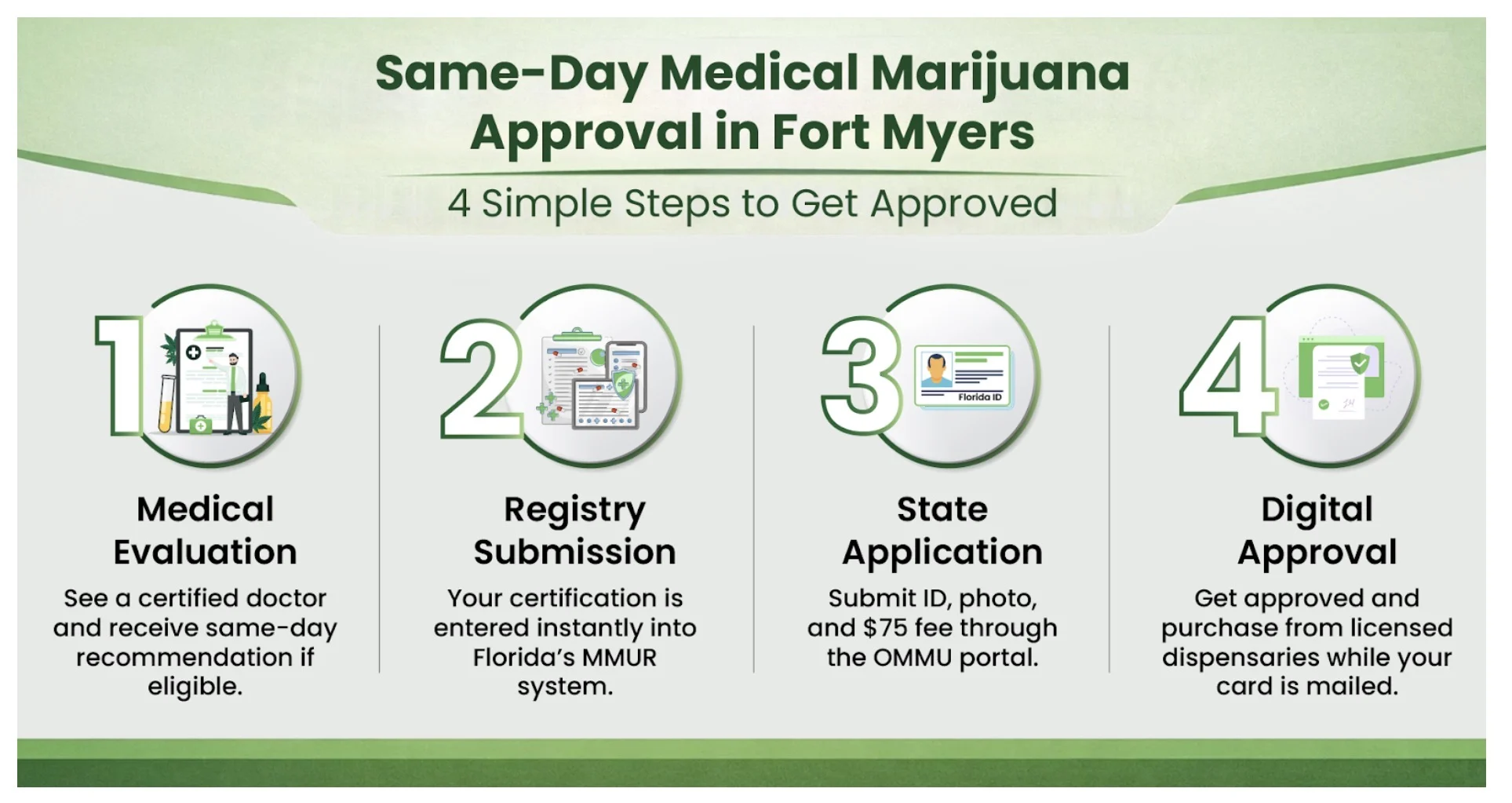
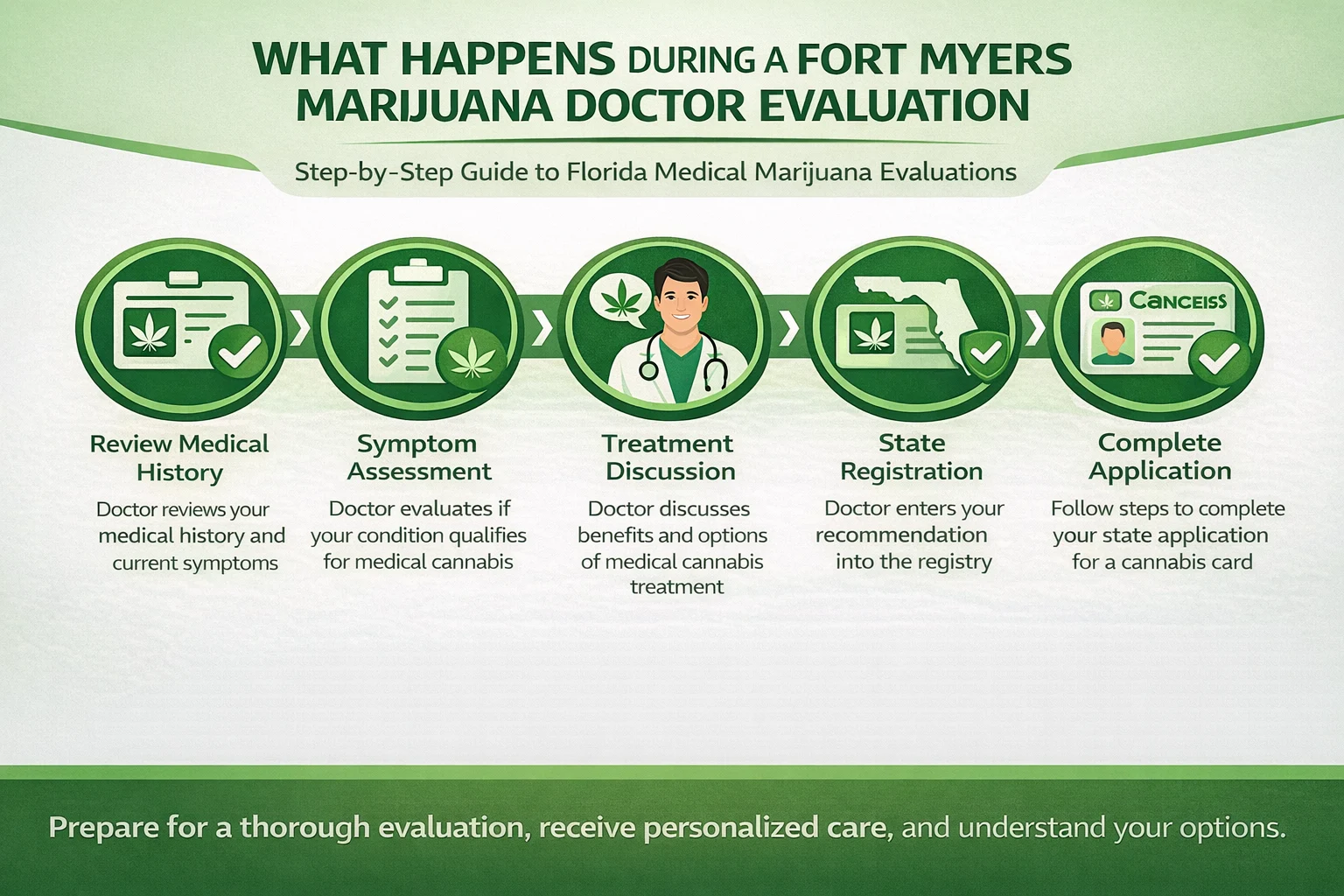
Medical cannabis now sits at the intersection of two worlds: the formal scientific world of clinical trials and the real-world experiences of millions of patients who use cannabis daily for chronic pain, PTSD, anxiety, neurodegenerative conditions, cancer symptoms, sleep disorders, and more.
Although the scientific community has produced meaningful research, clinical data often lags behind what patients actually use and experience. This gap affects treatment quality, patient safety, dosing guidance, and public understanding of cannabis as medicine.
Bridging these worlds is essential for building a modern, evidence-informed cannabis framework that truly serves patients.
Why Clinical Research Still Lags Behind Modern Cannabis Use
Decades of Regulatory Restrictions Limited Scientific Progress
For many years, cannabis was classified federally as a Schedule I substance, creating a bottleneck that affected nearly every part of the research pipeline. Even today, researchers face challenges accessing diverse, real-world cannabis products.
Background resources:
- NIH — https://www.nih.gov
- NIDA — https://nida.nih.gov
Studies often relied on cannabis materials that:
- Had lower potency than dispensary products
- Lacked modern terpene profiles
- Did not represent today’s ratioed tinctures, concentrates, or edibles
- Could not match the chemical diversity available to patients
This mismatch limits how well clinical results translate to real-world care.
Schedule your appointment today for a Medical Marijuana Card in Florida with My Florida Green’s certified doctors.
Real-World Cannabis Use Is More Complex Than Most Clinical Trials
Modern Products Have Surpassed Outdated Research Categories
Most published studies evaluate only a handful of variables (THC, CBD, or basic ratios). Meanwhile, real-world patients may choose from:
- Full-spectrum tinctures
- Minor cannabinoids like CBN, CBG, THCV
- High-THC concentrates
- Terpene-dominant formulations
- Edibles with varied onset times
- Nanoemulsified products
- Topicals, vaporizers, RSO, and more
These formats behave differently in the body, meaning clinical findings may only capture a fraction of how cannabis is experienced by patients.
Dosing Practices Differ Sharply
Clinical studies often rely on fixed dosing schedules. Real-world patients, however, use gradual titration, a process of adjusting dose and timing based on symptom relief, tolerance, and daily functioning.
This explains why medical guidance must often blend research with patient-reported outcomes.
Why Closing the Evidence Gap Matters For Patients
Patients Use Cannabis Alongside Other Medications
Real-world evidence shows many patients use cannabis to reduce reliance on opioids, benzodiazepines, NSAIDs, sleep medications, and antidepressants.
But without modern research on long-term interactions or optimal tapering strategies, clinicians often rely on:
- Observational data
- Small cohort studies
- Patient feedback
To review current study activity, see:
- ClinicalTrials.gov — https://clinicaltrials.gov
Veterans and Chronic Pain Patients Are Underrepresented in Trials
Some of the largest cannabis patient populations—especially veterans with PTSD and adults with chronic pain—remain underrepresented in controlled studies.
Research gaps include:
- Long-term outcomes
- Effects of high-potency formulations
- Benefits of multi-method dosing (oral + inhaled + topical)
- Impacts on sleep architecture, anxiety cycles, and flare-ups
More inclusive datasets are needed to support precise clinical guidance.
Structural Barriers That Slow Evidence Development
Restrictive Research Models
Traditional pharmaceutical-style research models struggle to study botanical medicine because:
- Cannabis contains hundreds of active compounds
- Terpene and minor cannabinoid interactions vary
- Real-world dosing is not linear
- Patient groups are diverse
- Individual responses can differ widely
More flexible research methods are required to reflect reality.
Lack of Longitudinal Observation
Most cannabis studies last weeks or months. Real patients often use cannabis for years.
Long-term data is essential to better understand:
- Tolerance pathways
- Cognitive and neurological impacts
- Cardiovascular trends
- Sleep outcomes
- Quality-of-life trends
- Dosing stability or evolution over time
For one example of foundational scientific review:
- National Academies of Sciences — https://www.nationalacademies.org
How Science and Real-World Use Can Finally Come Together
1. Allow Researchers Access to Real Dispensary Products
To generate meaningful insights, researchers need access to the same types of cannabis that patients use: modern flower, concentrates, edibles, tinctures, and full-spectrum extracts.
2. Build Patient Registries to Capture Real Outcomes
Just as oncology and cardiology use national registries, cannabis care can benefit from:
- Symptom-tracking datasets
- Dosing logs
- Side-effect trends
- Comorbidity profiles
- Longitudinal outcome data
This kind of aggregated data strengthens medical understanding far more quickly than sporadic clinical trials alone.
3. Use Hybrid Study Designs
A mix of clinical trials + observational studies allows science to retain rigor while reflecting real-world dosing patterns and patient priorities.
4. Improve Clinician Education
Many clinicians still lack training in:
- Endocannabinoid system fundamentals
- Terpenes and minor cannabinoids
- Drug–cannabinoid interactions
- Ratio selection
- Dosing strategies beyond fixed-schedule models
Updating medical education ensures safer and more effective patient guidance.
5. Integrate AI & Data Science
Large language models and clinical AI tools can help identify:
- Patterns in patient-reported outcomes
- Optimal dosing clusters
- Risk factors
- Medication interaction considerations
- Symptom-specific response trends
AI doesn’t replace clinical research — it amplifies it.
Ethical Considerations When Combining Science & Real-World Use
Avoid Overstating Benefits
Cannabis research is promising, but still evolving. Responsible communication requires:
- No claims of cure
- No guaranteed outcomes
- Clear acknowledgement of individual variability
- Emphasis on patient safety and thoughtful dosing
Protecting Patient Privacy
Any real-world data, registry, or observational study must operate under:
- HIPAA compliance
- Transparent data use
- Informed consent
- Encryption and security protocols
Medical ethics and patient trust must remain foundational.
Nick Garulay’s Vision — Building a Modern Understanding of Cannabis Medicine
Bridging the gap between clinical data and real-world cannabis use is not just an academic challenge — it’s a public health priority. Patients deserve guidance rooted in both evidence and lived experience.
By expanding research access, modernizing study design, improving clinician education, and incorporating real-world data, medical cannabis care can become more precise, more effective, and more responsive to the complex needs of patients.
If you are exploring whether medical cannabis may help you — or if your Florida Medical Marijuana Card is due for renewal — My Florida Green provides patient-first guidance and ethical support.
Thanks for reading — please care and share with your friends and family! Together, we can do so much better.
Learn more or get started: https://myfloridagreen.com.
If you’re already a patient and want to stay in good standing with Florida law, renewing your card early keeps your access uninterrupted.
Renew or update your information anytime. Click here to update.