Ongoing research on Medical Marijuana has unraveled the immense healing potential of this natural herb. There is a lot of research currently ongoing to understand the potential benefits and risks associated with using Marijuana for managing seizures. There is a lot of funding and effort to understand medical marijuana and epilepsy, and to determine the exact nature of its effect on controlling seizures and seizure-producing conditions.
Epilepsy is a common neurological disorder characterized by episodic seizures. They vary in severity and frequency, ranging from mild to severe. Epilepsy symptoms can bring serious challenges to the lives of patients. The causes of epilepsy are many, ranging from genetics to brain injuries. Epilepsy has a huge impact on the patient’s life and makes everyday routine tasks a challenge.
Many people in Florida today want to know how they can use medical marijuana for epilepsy. They want to know if epilepsy qualifies as a condition for which Marijuana can be taken legally. This article explores all the benefits of medical marijuana for epilepsy, and gives information on how to obtain a medical Marijuana card for eligible patients.
Schedule your appointment today for Medical Marijuana Card in Florida with My Florida Green’s certified doctors.
Understanding Epilepsy
Epilepsy is a neurological disorder characterized by recurring, of varying degree, intensity and severity. These seizures result from abnormal electrical activity in the brain and may cause:
- Loss of consciousness
- Uncontrolled muscle movements
- Confusion
- Staring spells
- Sudden behavioral changes.
- Physical injuries during seizures
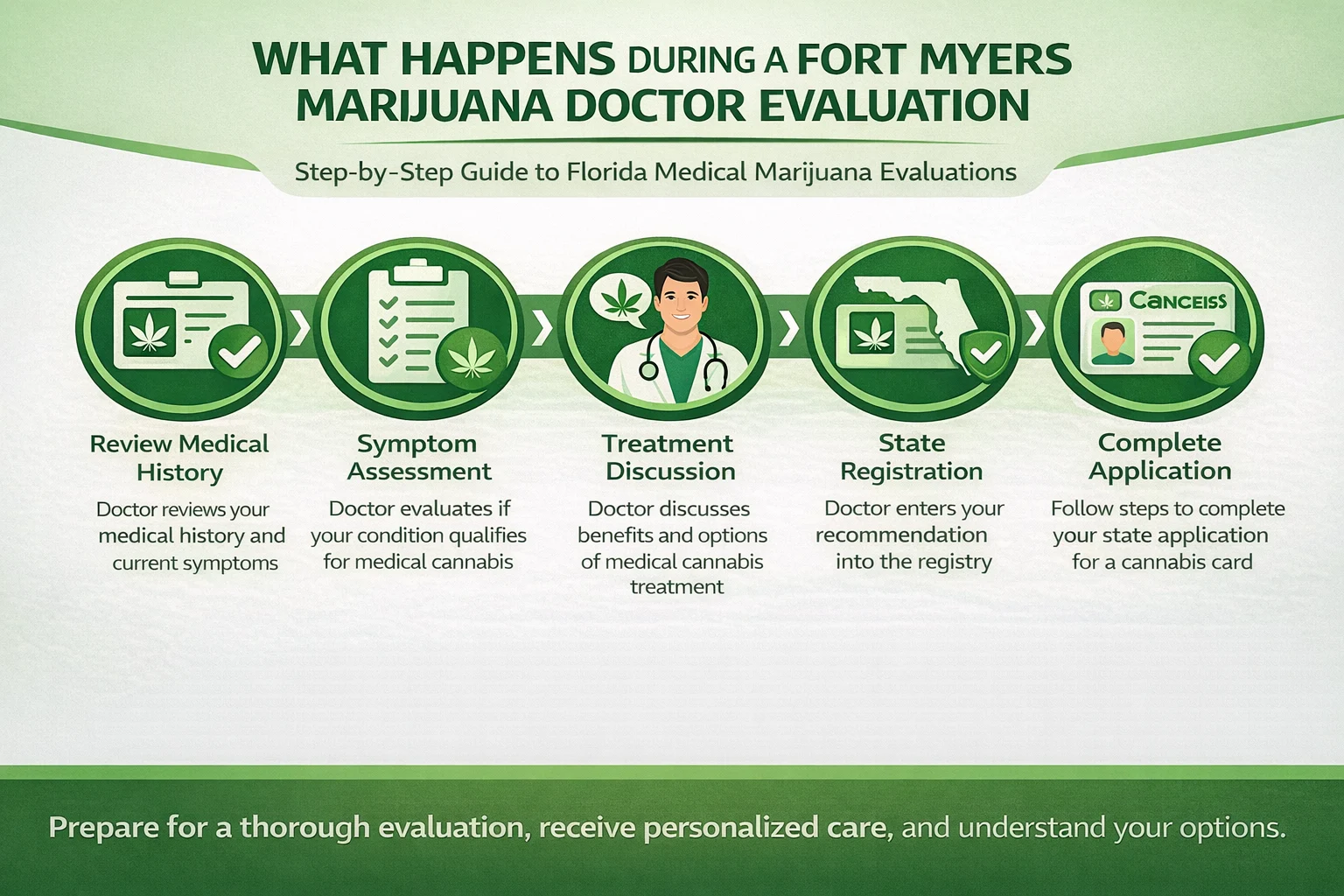
More than 3 million people in the U.S.live with epilepsy, and for many, traditional anti-seizure medications can be effective, but do not work for some. Hence, there has been ongoing research to understand the effects of marijuana on epilepsy. All patients must see a certified medical marijuana doctor in Florida who can evaluate their condition before recommending Cannabis.
How does Medical Marijuana help in managing epilepsy symptoms?
Medical marijuana has gained attention due to its potential anticonvulsant properties, especially CBD (cannabidiol), a non-psychoactive compound found in cannabis. Key ways cannabis may help epilepsy:
- Reducing seizure frequency
- Lowering seizure intensity
- Supporting better recovery after episodes
- Improving sleep and mood
- Enhancing quality of life in patients with drug-resistant epilepsy
Research expanded significantly after FDA approval of Epidiolex, a CBD-based medication shown to reduce seizures in childhood epilepsy syndromes such as Dravet and Lennox-Gastaut. Still more research is required to fully understand marijuana for epilepsy seizures and how it can help patients, without causing any harmful side effects.
How is Marijuana beneficial in managing epilepsy?
Epilepsy is included in the qualifying conditions for a medical marijuana card, because it has shown potential to manage seizures and associated symptoms like sleep, mood and stress.
1. Interaction with the Endocannabinoid System (ECS)
The ECS regulates brain activity, muscle control, mood, and nerve communication. CBD helps stabilize this system by modulating overactive neural firing.
2. Anti-Inflammatory and Neuroprotective Effects
Chronic inflammation in the brain can worsen seizure activity. Research has shown CBD’s potential in reducing neuroinflammation, hence supporting brain health.
3. Reduction of Excitatory Impulses
Seizures are mostly caused by excessive electrical activity. If the activity can be reduced, the seizures can be managed better. CBD has the potential to reduce overstimulation, which helps patients suffering from seizures.
4. Improved Mood
Patients suffering from epilepsy often have stress and anxiety, which comes with fear of the seizure, which can come anytime. Many patients also suffer from sleep issues, which further affects their mood. Medical marijuana helps improve sleep quality, which improves the overall mood of the patient.
The benefits of using Marijuana in the recommended dose have been demonstrated by physicians and clinical. These include:
- Fewer seizures in treatment-resistant epilepsy
- Less intense episodes when seizures occur
- Better tolerance compared to some conventional medications
- Reduced side effects such as sedation or cognitive impairment
- Improved appetite, sleep, and mood
- Enhanced daily functioning
While not a cure, medical marijuana can significantly improve the quality of life for many epilepsy patients.
What is the best Marijuana for Epilepsy?
It is important that Marijauna is always taken under the guidance of a State-certified doctor. They are the most experienced people to determine the best marijuana for epilepsy. Mostly products are dominant in CBD-rich, with low-THC recommended. Some Marijuana products are popular among patients, including:
- Tinctures & Oils (Most Common)– These offer fast absorption and precise dosing.
- Capsules – These are best used at night and offer consistent dosing. Suitable for long-term maintenance
- Edibles – These offer longer-lasting effects, but must be taken cautiously because the onset is slower. Only a certified Marijuana doctor can determine the best marijuana strains for epilepsy.
- Vaporisation (CBD) – These offer fast relief but are less commonly used for epilepsy
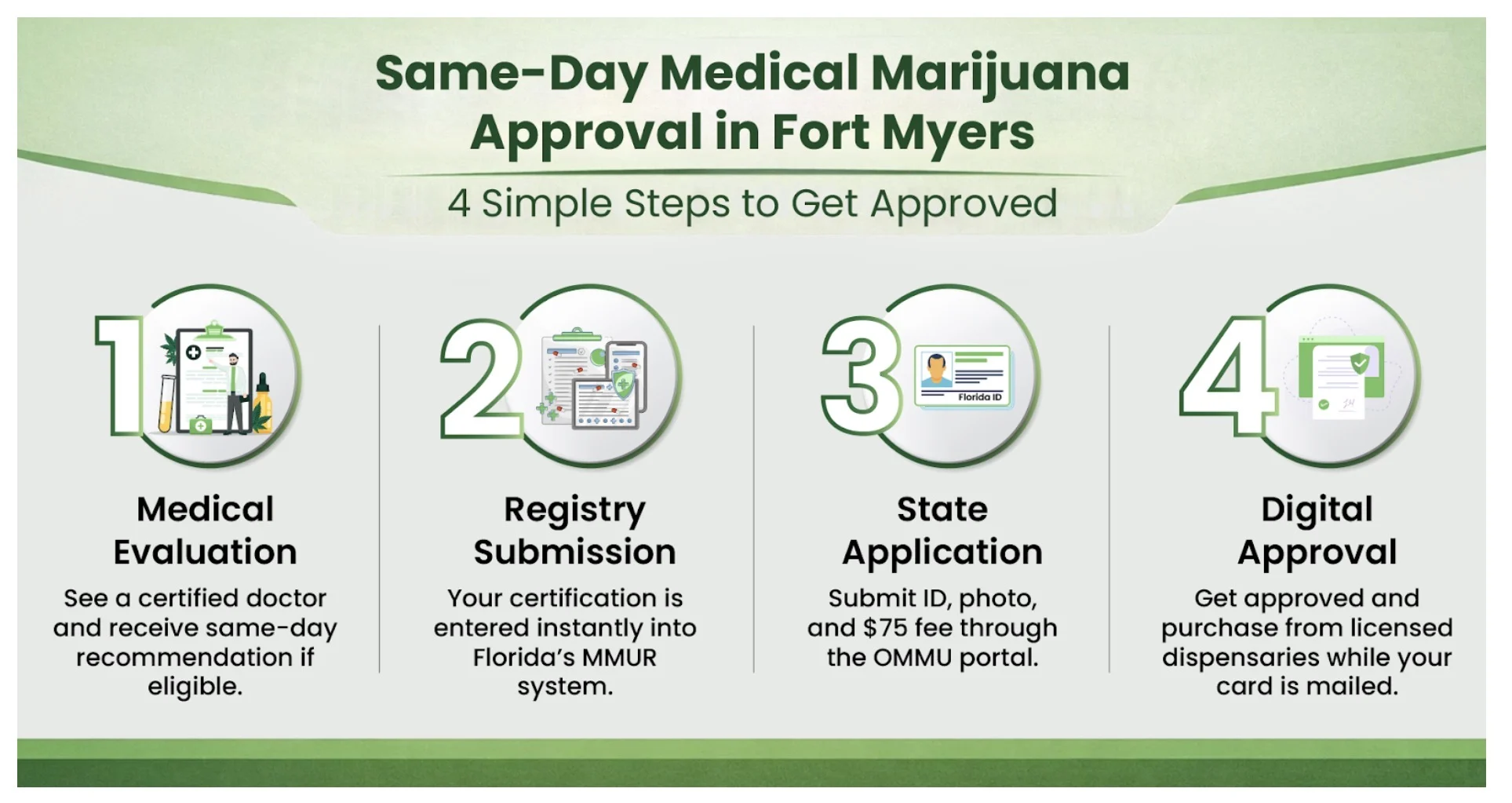
In Florida, epilepsy is an official qualifying condition for a medical marijuana card. Patients can see a qualified doctor, and once they get the approval from the expert, they can apply for a Medical Marijuana Card. The card allows patients to buy different marijuana products from licensed dispensaries.
Understanding Marijuana’s potential benefit in managing epilepsy
Marijuana has been the focus of debate and research for years, as a potential natural herb to manage epileptic symptoms. It has shown great potential when it is taken in the prescribed amount. With its neuroprotective, anti-inflammatory, and anticonvulsant properties, CBD-rich medical marijuana may help reduce seizure activity and improve quality of life.
For Florida patients, obtaining a medical marijuana card is straightforward, and epilepsy qualifies under state law. You can reach out to experts at My Florida Green, who make the certification process easy and efficient.
Frequently Asked Questions (FAQ)
1. Can I get medicinal marijuana in Florida for epilepsy?
Yes. Epilepsy is a recognized qualifying condition for a medical marijuana card in Florida. Adults and children may qualify after evaluation by a certified medical marijuana doctor.
2. Does marijuana help with epilepsy?
Research shows that CBD-rich medical marijuana can reduce seizure frequency and severity, especially in treatment-resistant cases. Individual results vary, and physician guidance is essential. More research is being conducted to fully understand Marijuana’s potential in managing epilepsy.
3. What are the best marijuana strains for epilepsy?
High-CBD, low-THC strains such as Charlotte’s Web, ACDC, and Ringo’s Gift are widely recommended for seizure management.
4. Is marijuana used to treat epilepsy in children?
Marijuana is not a treatment for epilepsy. However, it has shown benefits in managing pediatric epilepsy, especially products dominant in CBD. Children must be evaluated by 2 certified medical marijuana doctors, and only non-intoxicating CBD products are recommended.
5. Does THC help or worsen epilepsy?
Marijuana tends to affect everyone differently. Hence, it must be taken with caution and under the guidance of a doctor. Doctors usually recommend CBD-dominant products with low amounts of THC. High THC levels can trigger anxiety or seizures in others.